Uploaded documents can be shared based on the number of copies available by the personas who has the necessary permissions to do so.

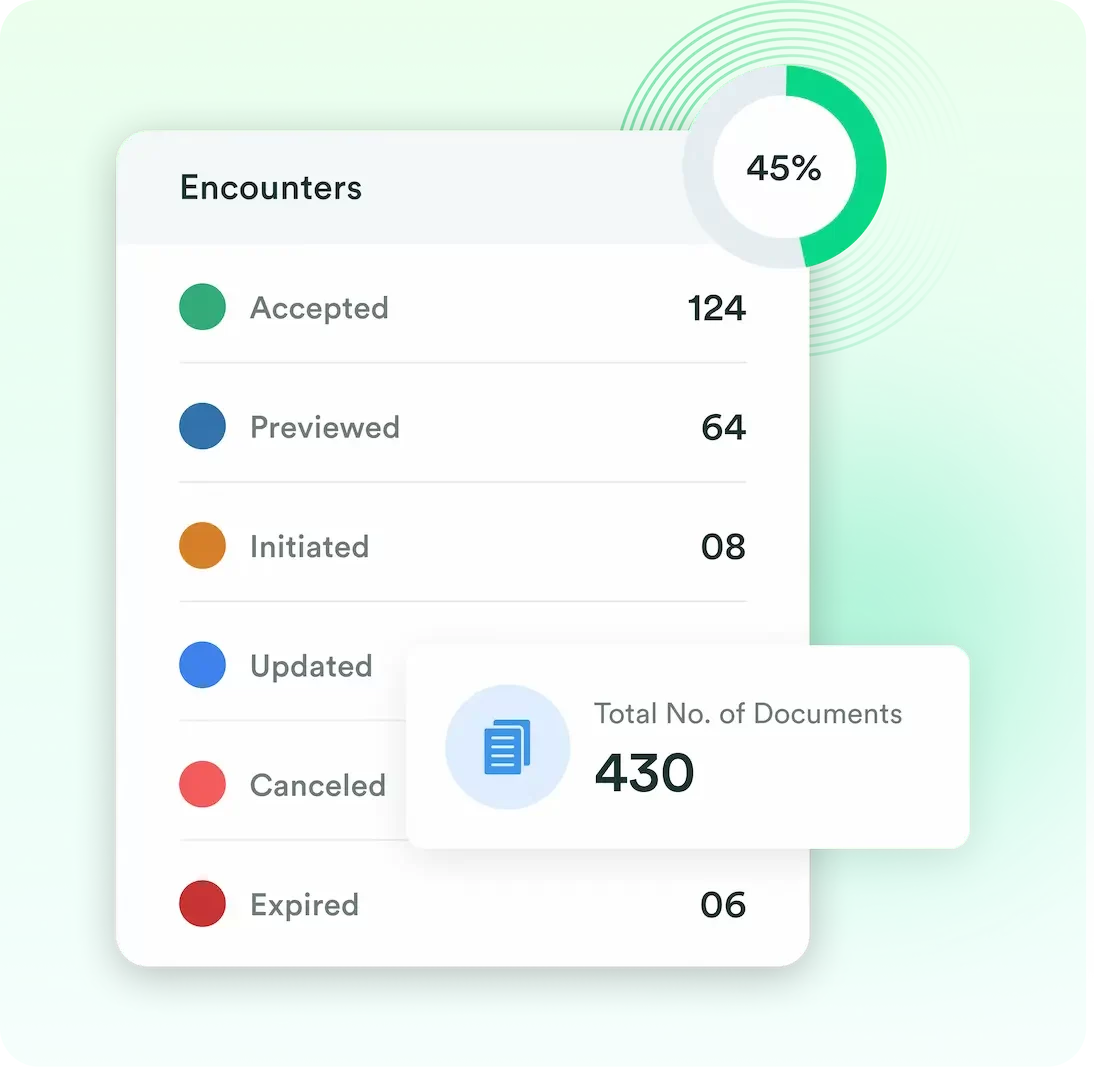

Documents, Encounters, Staff, Company and End User’s data is collected and can be viewed by authorized personnel.

We offer advanced analytics that provide valuable insights into user engagement. This enables monitoring of document interaction in real-time, ensuring efficient and secure information sharing.




Patrick Reilly
CEO, phacMI
In a recent conversation with Darshan, I was pleased to hear about a new product called “Ceres”. He plans to offer pharmaceutical companies this product in order to fill a current void and/or provide a better solution than what is currently being utilized regarding compliant documentation of information being shared with HCPs. Ceres seems to offer not only a compliant but also a flexible, efficient, and user friendly experience solution beyond what’s currently being utilized today

Seth Whitelaw
CEO, Whitelaw compliance group
One of the biggest challenges, I faced as a compliance officer was keeping track of multiple, integrated regulatory requirements, especially those involving the Sunshine Act and FDA requirements. I’m really excited about the potential of Ceres to integrate those disparate requirements and track solutions to address them. If you face similar challenges, I urge you to check this innovative solution out

Patrick Reilly
CEO, phacMI
In a recent conversation with Darshan, I was pleased to hear about a new product called “Ceres”. He plans to offer pharmaceutical companies this product in order to fill a current void and/or provide a better solution than what is currently being utilized regarding compliant documentation of information being shared with HCPs. Ceres seems to offer not only a compliant but also a flexible, efficient, and user friendly experience solution beyond what’s currently being utilized today

Seth Whitelaw
CEO, Whitelaw compliance group
One of the biggest challenges, I faced as a compliance officer was keeping track of multiple, integrated regulatory requirements, especially those involving the Sunshine Act and FDA requirements. I’m really excited about the potential of Ceres to integrate those disparate requirements and track solutions to address them. If you face similar challenges, I urge you to check this innovative solution out
© 2022 Ceres. All rights reserved